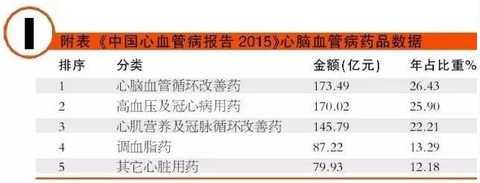
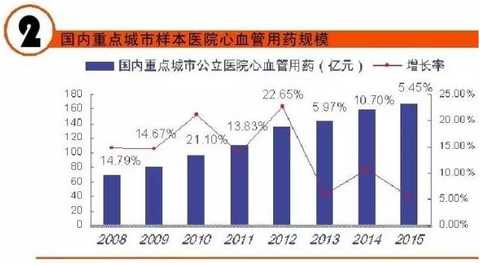
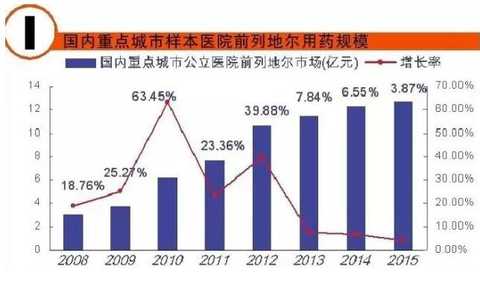
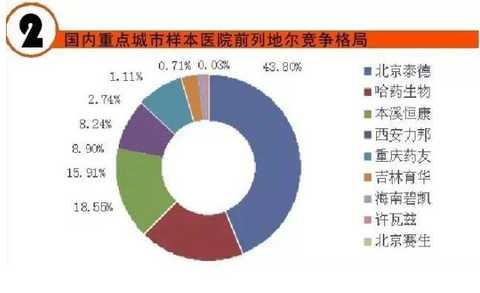
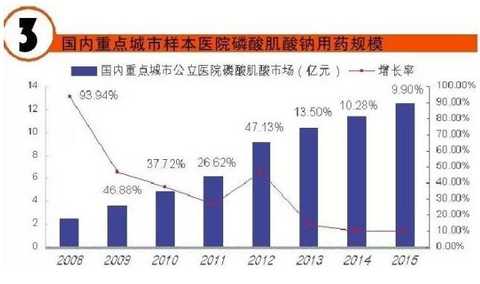
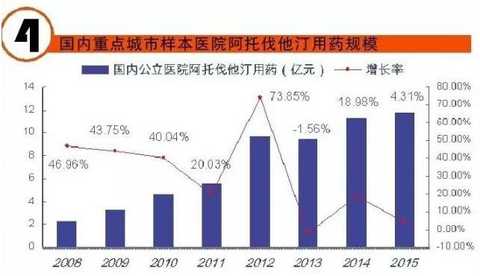
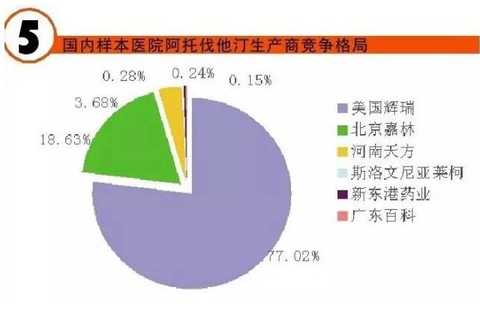
[Huicon Pharmaceutical Industry Network] According to the statistics of the 2015 Cardiovascular Disease Annual Report, the total purchase amount of hospitals in China with more than 100 beds is 614.76 billion yuan, of which the total amount of cardiovascular and cerebrovascular diseases is 65.645 billion yuan, accounting for 10.67%. In 2020, it will break through the 100 billion yuan mark. Cardiovascular disease is the world's first cause of death; it has become China's first chronic disease with high prevalence, high disability, high mortality, high medical risk and high medical expenses, which seriously affects the body and mind of all human beings. health. According to the World Health Organization, more than 17 million deaths are caused by heart disease worldwide each year. It is estimated that by 2020, the number of deaths caused by cardiovascular disease worldwide will increase to 25 million, of which 76% occur in developing countries with insufficient awareness and inadequate medical conditions. Cardiovascular disease According to the National Cardiovascular Disease Report 2015 compiled by the National Center for Cardiovascular Diseases, there are 290 million cardiovascular patients in China, and the annual increase rate is 17 million. More importantly, the mortality caused by cardiovascular disease in China is high, and the number of people who die of cardiovascular disease each year is 3.5 million. Among them, the mortality rate of acute myocardial infarction accounts for 16%, and the incidence of the disease has become younger; it is serious. One of the major diseases that threaten human health. It is worth mentioning that in the rural areas after the rich, the incidence of cardiovascular disease and mortality are higher than the city. Most people suffer from high blood pressure, hyperlipidemia, and moderate cardiovascular disease, which require lifelong treatment, which has led to a significant increase in social security medical insurance spending in China. According to the statistics of the 2015 Annual Report of Cardiovascular Disease, the total purchase amount of medicines in hospitals with more than 100 beds in China was 614.76 billion yuan. Among them, the total amount of medicine for cardiovascular and cerebrovascular diseases was 65.645 billion yuan, accounting for 10.67%. Research and analysis showed that 2020 The domestic cardiovascular market will break through the 100 billion mark. The top 4 drugs that have been spent more than 5 billion yuan are: cardiovascular and cerebrovascular circulation improving drugs, hypertension and coronary heart disease drugs, cardiotrophic drugs and coronary circulation improving drugs, and blood lipid regulating drugs, which together account for the total amount of cardiovascular and cerebrovascular diseases. 87.82% (see attached table for details). Hospital terminal to 16.68 billion Studies have shown that the mortality rate of cardiovascular and cerebrovascular diseases in China is higher than that of developed countries such as Europe, America and Japan. If the government does not take active measures, in the past 10 years, domestic non-infectious chronic diseases such as cardiovascular disease, stroke and diabetes will cause about 600 billion yuan in economic losses, making the social security medical insurance fund unable to make ends meet. According to CFDA Southern Pharmaceutical Economic Research Institute Punctuation Information Company "China City Public Hospital Chemical Drug Terminal Monitoring and Analysis System" (HDM System) for Beijing, Shanghai, Guangzhou, Hangzhou, Chengdu, Wuhan, Chongqing, Jinan, Nanjing, Changsha, Shenyang, Tianjin Statistics on 16 cities including Xi'an, Zhengzhou, Harbin and Shijiazhuang showed that the cardiovascular drug treatment market in public hospitals in 2015 was 16.673 billion yuan, an increase of 5.45% over the previous year, including cardiovascular and anticoagulant drugs. . Although the growth rate of the cardiovascular and cerebrovascular market has slowed down in the past two years, the amount of medication has continued to rise. Comprehensive sequencing shows that TOP10 cardiovascular and cerebrovascular drugs are clopidogrel, alprostadil, creatine phosphate, atorvastatin, complex coenzyme, rosuvastatin, amlodipine, butylphthalide, cinepazide and nitrate. Bendipine. The amount of TOP10 drug used was 8.241 billion yuan, accounting for 49.13% of this large class of drugs. Since the mid-to-late 1980s, with the development of global medical treatment technology, the early mortality rate of patients with ST-segment elevation myocardial infarction (STEMI) has been decreasing year by year. But overall, the incidence and mortality of acute myocardial infarction remains high. Extend > "Four Tigers" join forces to encircle modern civilization diseases The rise of antiplatelet and anticoagulant drugs further expands the cardiovascular disease market. It is worth noting that the new drug release technology and compound preparations will be the highlights of research and development during the 13th Five-Year Plan period. The European Society of Cardiology (ESC) annual meeting issued the "Guidelines for the diagnosis and treatment of ST-segment elevation acute myocardial infarction", which is an effective strategy to guide clinical emergency treatment, reperfusion therapy, direct percutaneous coronary intervention and thrombolytic therapy. Although direct percutaneous coronary intervention is the first choice, medical treatment is still one of the important links due to various medical conditions, individual physical conditions, and time of onset of symptoms. The main medical treatment for acute myocardial infarction (AMI) is thrombolysis. Traditional clinical drugs are nitroglycerin, urokinase, heparin, streptokinase and recombinant tissue plasminogen activator. In recent years, the rise of antiplatelet drugs and anticoagulant drugs has further improved the anti-thrombosis and anticoagulation markets. Especially in recent years, with the advent of prasugrel, ticagrelor, rivaroxaban, dabigatran etexilate, and apixaban, the clinical means of antithrombotic and anticoagulation have been further increased. 1. Clopidogrel wins the domestic market In the late 1990s, the US FDA approved clopidogrel developed by Bristol-Myers Squibb (BMS) for the prevention and treatment of diseases such as atherosclerotic disease, myocardial infarction, stroke and peripheral vascular disease. A landmark variety. Clopidogrel and aspirin low-dose enteric-coated tablets are known as anti-platelet, gold standard treatment for preventing blood coagulation, and are drugs for preventing stroke and myocardial infarction. Clopidogrel is marketed by Bristol-Myers Squibb and Sanofi-Aventis under the trade name "Plavix". Clopidogrel was developed and marketed earlier in China. By the end of 2015, 16 raw material drug production lines have been built in China. In 2000, CFDA first approved the registration of clopidogrel bulk drug and its tablets in Shenzhen Xinlitai Pharmaceutical Co., Ltd. under the trade name “Taijiaâ€. In 2001, Sanofi-Aventis' clopidogrel was approved for sale in China under the trade name “Poliviâ€; in December 2014, Iceland's Actavis Group PTCehf clopidogrel tablets were approved by the CFDA to enter the Chinese market. Driven by the original research drugs and domestically produced drugs, it has become a fast-growing variety and is now a bright spot on the market. According to data from the HDM system, in 2015, the clopidogrel market in public hospitals in key cities in China was 1.439 billion yuan, an increase of 8.4% over the previous year. Among them, Sanofi Aventis's Polivi accounted for 59.40%, Shenzhen Xinlitai's Taijia occupied 36.15%, and Lepu Pharmaceutical's Shuike accounted for 4.46%. In 2015, the overall sales volume of domestic clopidogrel tablets reached 9.244 billion yuan, ranking the third in the domestic pharmaceutical market, up 3.62% year-on-year. Under the factors of strong domestic cardiac drug market, clopidogrel is expected to surpass sodium chloride. Injection and glucose injection have won the domestic pharmaceutical market. 2, the former Listil monopoly is strong Alprostadil is a drug for refractory cardiovascular and cerebrovascular ischemic diseases and chronic arterial occlusive disease. It can effectively improve cardiovascular and cerebrovascular microcirculation disorders, organ transplantation and postoperative anti-embolism. At present, there are 33 productions in China. The main preparations are: injection, powder injection, urethral suppository, dry emulsion for injection. Ordinary powder injections have a large dosage, and the active ingredients reaching the point of action are insufficient, which affects the clinical promotion and the expansion of the terminal market. Under the background of the continuous development of new drug release technology, the alprostadil lipid microsphere targeting agent developed by Beijing Tide has dominated the market. Modern pharmacy research and development of vascular embolization microsphere preparations is a long-acting sustained release dosage form for the development of topical administration. The lipid microsphere carrier targeting preparation is a microsphere dispersion system formed by encapsulating prostaglandin E1 in lipid microspheres by a proprietary technique, homogenizing the high pressure emulsion, and encapsulating the lipid microspheres into a 0.2 micron particle size. . Under the action of human body physiology and physiology, the fat particles are used as a drug carrier to efficiently transport prostaglandin E1 to the lesion site, so that the micron-sized drug can be released at the required site, and the drug can be automatically concentrated and concentrated on the diseased organ and cell tissue after administration. . The microparticle carrier can not only protect the activity of the drug, but also can transport the drug to be transported to the lesion site to release the effect; the local effective concentration of the drug is increased, and the maximum effect is exerted with less dose. Alprostadil is an important variety in the cardiovascular medicines of the sample hospitals, and it is also a rapidly rising market, with a strong monopoly. Under the impetus of the new drug release technology, aldiendil was the first listed city of the city's key cities in 2015. The market was 1.275 billion yuan, an increase of 3.78% over the previous year. Among them, Beijing Tide's brand drug “Kaishi†accounted for 43.8%, and other manufacturers accounted for 56.20%. 3, sodium creatine phosphate products self-improvement Sodium creatine phosphate is a novel, superior cell protectant with high energy excitation. It is a drug that protects against abnormal myocardial metabolism in an ischemic state during cardiac surgery. At the end of the 20th century, Alpha Weissman's sodium creatine phosphate was launched in China under the trade name "Neoton". In 2012, CFDA approved five listings including Changchun Yinglian Biotechnology, Haikou Keli Pharmaceutical, Harbin Bolai Pharmaceutical, Beijing Lixiang Pharmaceutical and Hebei Tiancheng Pharmaceutical. Creatine phosphate is a self-contained active substance in the body that is specifically designed to replenish energy for ATP, which is the most important source of energy in any cellular metabolic process. Exogenous sodium creatine phosphate is a cardioprotective drug that can be used as an important adjuvant for reducing ischemic myocardial damage, ventricular arrhythmia and improving failure during cardiac surgery. Especially after the widespread application of myocardial infarction thrombolysis and percutaneous transluminal coronary angioplasty (coronary stent) and coronary artery bypass grafting, the rapid growth of the sodium creatine phosphate market has been driven. According to data from the HDM system, in 2015, the market for phosphocreatine in public hospitals in key cities in China was 1.258 billion yuan, an increase of 9.9% over the previous year. Alpha Weissman's imported brand, Liertong, only accounted for 2.59%; while domestic brand medicines accounted for 97.41%, of which Jilin Yinglian Bio's "Nasdaq" accounted for 37.82%, and Haikou Keli's "Weijia Neng" accounted for 29.66%, Harbin Laibotong accounted for 25.41%, Hebei Tiancheng's "powerful" accounted for 4.36%, Beijing Lixiang's "Jinbo" occupied 0.16%, it can be said that sodium creatine phosphate has been localized. 4, atorvastatin is unstoppable Atorvastatin is the fourth drug in the domestic cardiovascular market. It is the leading drug in the blood lipid regulating market in China, occupying half of the blood lipid regulating market. According to data from the HDM system, in 2015, the amount of atorvastatin in public hospitals in key cities in China was 1.18 billion yuan, a year-on-year growth rate of 4.31%. The brand of market competition is Lipitor of the United States, which accounts for 77.02% of the market share; Beijing Jialin Pharmaceutical Co., Ltd.'s first generic drug "Ale" accounted for 18.63%. After the market cultivation "Ale" has become the third place in the domestic statin market. In addition, Tianyou Pharmaceutical's “Youjia†accounted for 3.68%, and other companies sold atorvastatin accounted for 0.67%. According to domestic authoritative data, in 2015, the overall market of atorvastatin in China reached 8.319 billion yuan, an increase of 7.97% over the previous year. In the sample hospitals, atorvastatin has been a rapidly growing variety, and is also significantly higher than the overall domestic market growth rate. It is predicted that 2017 will break through the 10 billion mark and compete with clopidogrel. The new drug release technology and compound preparations will be the highlights during the 13th Five-Year Plan period. The atorvastatin combination formulation development pipeline is equally vibrant. The atorvastatin combination that has entered the clinical stage at this stage includes: atorvastatin + amlodipine tablets, atorvastatin + toseppe cloth, atorvastatin + levamlodipine tablets. Editor in charge: Yan Wenqian Men'S Jacket,2020 New Denim Jacket,Popular Jeans Jacket,Cotton Mens Jean Jacket Shaoxing Weihui International Trade Co.,Ltd. , https://www.weihui-fabric.com